Experts in the chemical and pharmaceutical development of peptide-based drug products and finished products up to pharmaceutical certification
+ 15 years
<p>of experience as a peptide CDMO</p>
500 m<sup>2</sup>
<p>of GMP laboratories</p>Know how

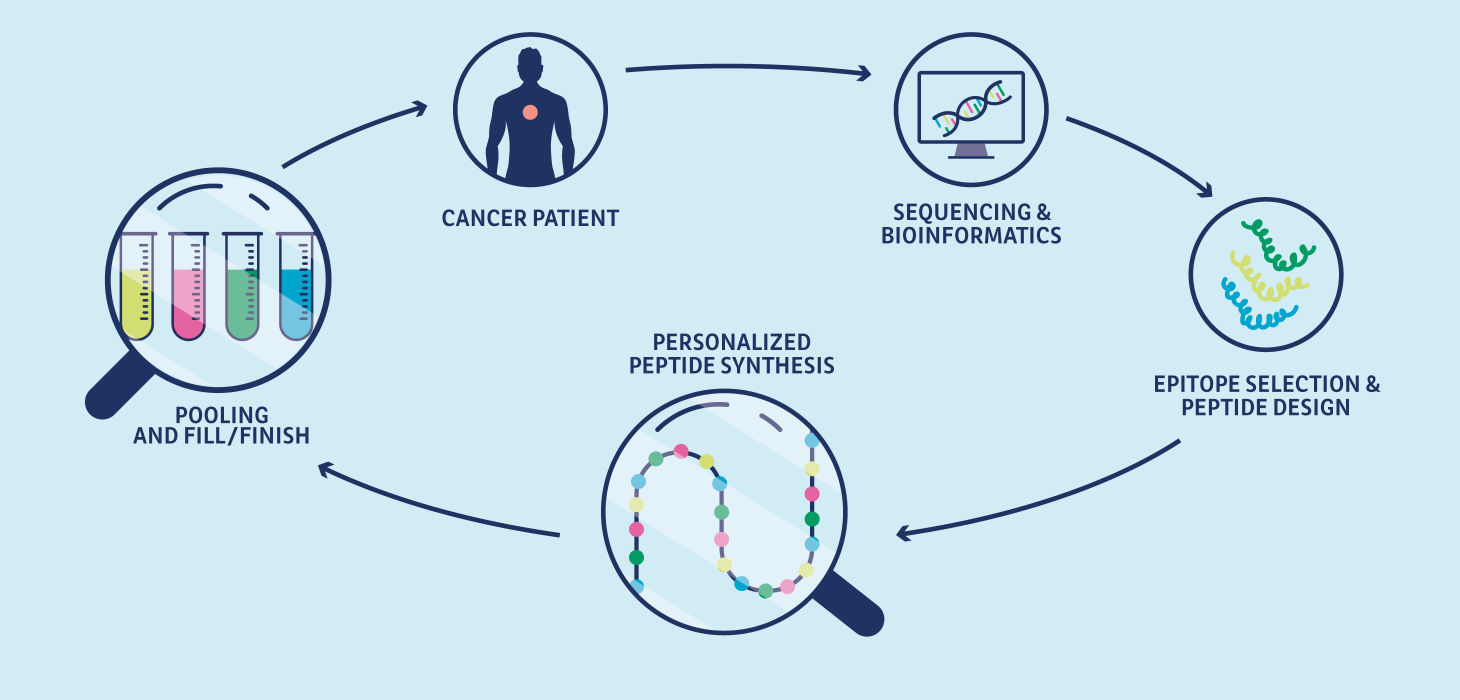
<p><strong>From chemical conception to clinical batches</strong></p>
<ul>
<li>Process development</li>
<li>Analytical development</li>
<li>Bulk or aliquoted technical batches</li>
<li>Pre-formulation</li>
<li>Manufacturing of API batches for tox studies</li>
<li>Manufacturing of API batches for clinical studies</li>
<li>Coordination of the manufacturing of finished products for clinical trials</li>
<li>Pharmaceutical certification for EU clinical trials</li>
</ul>
Expertise

<ul>
<li>Short peptides</li>
<li>Complex peptides</li>
<li>Long peptides (up to 150 mer)</li>
<li>Conjugated peptides (KLH, CRM197, Fab, PEG)</li>
</ul>
The teams work alongside our customers to find innovative and adapted solutions that guarantee the success of their pharmaceutical manufacturing projects.
Production means

<ul>
<li>Solid Surface Peptide Synthesis Platform (SPPS) for Process Development</li>
<li>Analytical department for quality control and development of analytical methods for chemical and biological compounds</li>
<li>Room dedicated to non-aseptic packaging of experimental batches</li>
</ul>

<ul>
<li>GMP production plant in controlled atmosphere zone for API manufacturing
<ul>
<li>Synthesis / Cleavage</li>
<li>Purification</li>
<li>Lyophilization</li>
<li>Sterilizing filtration</li>
<li>Aliquoting under laminar flow</li>
<li>Final lyophilization</li>
</ul>
</li>
<li>Analytical department for quality control, Qualification / Validation of analytical methods for chemical and biological compounds</li>
<li>Network of European partners for fill and finish activities:
<ul>
<li>Production of clinical batches in sterile environment</li>
<li>Packaging in therapeutic units</li>
<li>Pharmaceutical certification of batches for clinical trials</li>
</ul>
</li>
</ul>